Each year, billions of pounds of flame retardant additives are used throughout the world.
A variety of flame retardant chemistries, packages, or systems can be deployed in different polymers depending on the requirements of individual end-use applications.
Some polymers are inherently flame retardant. Other polymers — including nylons, polyesters, polypropylenes and many other useful and cost-effective materials — are not. They must be modified to achieve the proper level of fire resistance through the use of flame retardant additives.
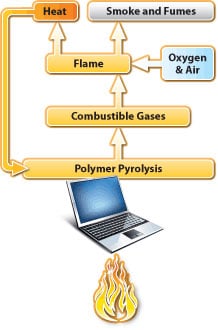
Mechanisms for flame retardance in plastics
Vapor Phase Inhibition
During combustion, flame retardant additives react with the burning polymer in the vapor phase disrupting, at a molecular level, the production of free radicals and shuts down the combustion process. This mechanism is commonly used with halogenated flame retardant systems.
Solid Phase Char-Formation
Char-forming flame retardant additives react to form a carbonaceous layer on the material’s surface. This layer insulates the polymer, slowing pyrolysis, and creates a barrier that hinders the release of additional gases to fuel combustion. This method is commonly deployed by non-halogen systems using phosphorous and nitrogen chemistries.
Quench & Cool
Hydrated minerals make up a class of halogen-free flame retardant systems commonly used for extruded applications like wire and cable. These systems use an endothermic reaction in the presence of fire to release water molecules that cool the polymer and dilute the combustion process.
RTP Company’s material engineers are well versed in both halogenated and halogen-free flame retardant technologies and can use their expertise to apply the proper material solution to your application.